The structure of metals The arrangement of the atoms Metals are giant structures of atoms held together by metallic bonds. For metals a distinction is made between the primary microstructure and the secondary microstructure.
6 4 Crystal Structures Of Metals Chemistry Libretexts
An architectural structural mechanical or electrical plan elevation or section indicating in isometric or in axonometric perspective the detailed location dimension quantity or extent of material product or member to be furnished.

. What fourth period element is represented by the dot structure. Strength ductility high melting point thermal and electrical conductivity and toughness. The structure of metals consists of layers of metal ions.
Structures of Metals and Ceramics STUDY PLAY Crystalline describes a material in which the atoms are situated in a repeating or periodic array over large atomic distances. Assumed premise Question 4 Not yet answered For the following argument you are given the structure of the. What is the term that best describes the following definition.
Mn Fe Co Ni Cu. A Li b Na c Rb d F e I 4. The crystal structure of harder metals such as antimony and bismuth makes it more difficult to press atoms into new positions without breaking.
Metals are elements that lose electrons easily that are lustrous reflective malleable can be molded into other shapes and are good conductors of heat and electricity. This is why metals are good conductors of electricity. Giant implies that large but variable numbers of atoms are involved - depending on the size of the bit of metal.
If heat is removed from the molten metal the energy of the constantly moving atoms in it decreases. Which element has the largest atomic radius. These layers can slide over each other when a force is applied.
Metals are crucial to. This means that the layers of. Metal Groupings on the Periodic Table alkali metals alkaline earth metals.
Main group elements that are metals usually _____ one or more electrons to form _____which have a _____ charge. Metals usually have what type of structure crystalline Which of the following material types has the simplest crystal structure metal The closest packed most densely packed metallic crystal. Most pure metals naturally adopt one of these three closest packing arrangements.
A substance with high electrical conductivity luster and malleability which readily loses electrons to form positive ions cations. Material Science and Engineering Chapter 3. The structure of a solid metal consists of closely packed metal ions arranged in a regular way to form a metallic lattice.
Updated on September 22 2019 The definition of metal. What types of bonds are shown in the lewis structure of BCl3. Metals account for about two thirds of all the elements and about 24 of the mass of the planet.
Cubic close packing is defined as a crystalline structure in which planes of closely packed atoms or ions are stacked as a series of three alternating layers of different relative orientations. This is because the rows of atoms in the metal dont line-up. A tapered pin used to align holes in steel members to be connected.
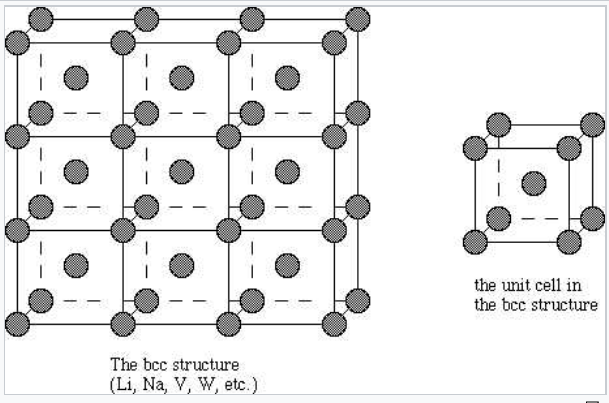
In that crystal metal atoms occupy the eight corners of a cube along with one atom in the very center. Crystalline materials include metals ceramics and parts of some polymers opposite of noncrystallineamorphous crystal structure. Metals are otherwise defined according to their position on the Periodic Table.
They are all around us in such forms as steel structures copper wires aluminum foil and gold jewelry. Which term best describes an orbital. The coordination number of each atom in the body-centered cubic structure is 8.
On the far left is the body-centered cubic bcc structure. Psychology questions and answers. The term which best describes the crystalline substance that results when a large number of metal atoms transfer electrons to a large number of non-metal atoms is.
Typically the atoms of metals contain less than half the full complement of electrons in their outermost shell. A d-transition metals b representative elements c metalloids d alkaline earth metals e halogens 3. In other words more grain boundaries exist which are areas where atoms are not as strongly connected.
Explaining properties of metals The giant structure of metals and metallic bonding explain their properties. Select the term best describing the series of elements. Alkali metals acquire a noble gas electron configuration by a.
Metals are usually crystalline solids. E Group VIIB and Period 4 2. Metals are widely used because of their properties.
A model for the structure of metals curriculum-key-fact. This page decribes the structure of metals and relates that structure to the physical properties of the metal. In most cases they have a relatively simple crystal structure distinguished by a close packing of atoms and a high degree of symmetry.
Also called Spud Wrench. The electrons from the outer shells of the metal atoms are delocalised and. Metals consist of giant structures of atoms arranged in a regular pattern.
The statement or proposition that logically follows from the previous propositions. When considering the composition of different types of unit cells of metals we find that simple cubic unit cells _______. Gaining two electrons.
Primary structure The primary structure is formed naturally during the solidification of a melt and is also called the casting structure.

Metallic Bond Properties Examples Explanation Britannica

Crystalline Vs Amorphous Solids Animation Solid State Class 11 12 Chemistry Digital Kemistry Youtube Chemistry Covalent Bonding Crystalline Solid

Metal Fabrication 101 Techniques Types Building Applications Metal Fabrication Infographic Metal Buildings

0 Comments